Abstract
Abstract
Background: Thalassemia major (TM) is a fatal genetic disease currently only curable with allogeneic stem cell transplantation, which is limited by the lack of suitable donors. For patients lacking matched related donors, unrelated donor peripheral blood stem cell transplantation (UD-PBSCT) is increasingly being utilized. Despite this success in leukemia patients, UD-PBSCT is not widely practiced in thalassemia patients because of higher graft-versus-host disease (GVHD) risk and increased transplant-related mortality (TRM).
Methods: The purpose of the study is to assess the safety and efficacy of UD-PBSCT for patients with thalassemia major. The trial was designed as a prospective, open-label, single-center clinical protocol, where 53 TM patients were enrolled between March 2013 and April 2017 and transplanted with peripheral blood stem cell (PBSC), from an HLA-matched unrelated donor. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University and was registered at the Chinese Bone Marrow Transplant Registry (CBMTR). The primary end point was 2-year thalassemia free survival (TFS). Secondary end points included 2-year overall survival (OS), the cumulative incidence of GVHD, transplant related mortality (TRM), graft rejection (GF). The conditioning regimen were: 1) busulphan (BU) 1mg/kg given intravenously (IV) four times per day for 4 days (day -9 to day -6); 2) fludarabine (FLU) (50mg/m2/day) given IV for 3 days (day -12 to day -11); 3) cyclophosphamide (CTX) (50 mg/kg/day) given IV for 4 days (day -5 to day -4); 4) anti-thymocytes globulin (ATG, Genzyme ) (2.5 mg/kg/day) given IV for 4 days (days -4 and day -1). All patients were placed on 30 mg/kg hydroxyurea orally once daily for 2-3 months before transplantation. GVHD prophylaxis consisted of a combination of cyclosporin A, methotrexate and mycophenolate mofetil regimen.
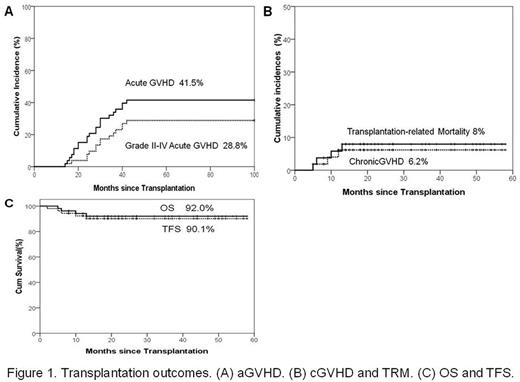
Results: Data cut off for survival follow-up was June 30, 2017. The median follow-up time was 22 months (range, 4 months -58 months). The median age of patients was 5 years (range, 2-13 years). The median numbers of MNCs and CD34 cells infused per graft were 17.6 ×108/kg (range, 10.0 to 34.6)and 9.2×106/kg (range, 3.5 to 32.9), respectively. A total of 52 (98.1%) patients achieved sustained myeloid engraftment. The median time of absolute neutrophil count engraftment was 11(range 9-15) days. The median time of platelet engraftment was 13(range, 9 to 35) days. The rate of Graft rejection was 1.9%. The cumulative incidence of acute (a)GVHD and grade II-IV aGVHD were 41.5% (95% confidence interval [CI], 29.1 to 56.2) and 32.0% (95% CI, 21.1 to 48.6) , respectively . The chronic (c)GVHD and extensive cGVHD were 6.2% (95% confidence interval [CI], 2.1 to 16.6) and 2.2% (95% CI, 0.3 to 15.1), respectively. The cumulative incidence of TRM at 2 years was 8.0% (95% CI, 3.1 to 20.4). The OS and TFS at 2 years were 92.0% (95% CI, 87.8 to 100) and 90.1% (95% CI, 85.0 to 99.6), respectively. (Figure 1)
Conclusion: Our results demonstrate that MUD-PBSCT in TM yields excellent engraftment rate and TFS when the protocol consisted of busulphan, cyclophosphamide, fludarabine and antithymocyte globulin was used, and suggest that patients without an available HLA-compatible sibling but who have well-matched unrelated donors should also be considered for MUD-PBSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal